Europe
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

UK MHRA’s Strategic Approach To AI Will Be ‘Proportionate’ And Fit Well Internationally
UK pursues light-touch regulation as it forges ahead with its own approach to regulating AI but with one eye on international convergence.

Global Medtech Guidance Tracker: April 2024
Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Thirty-five documents have been posted on the tracker since its last update.
.jpg?rev=dadcd0227e7c44faaefe9d1648bf0675&w=350&hash=2FDE0F9D94F05FD117261BE726C9DE37)
Front Line Medical Technologies Announce CE Mark For COBRA-OS
Front Line Medical Technologies Inc.'s new CE marking expands access of its occlusion device COBRA-OS to medical providers in Europe. Adam Power, chief medical officer at Front Line Medical, talked to Medtech Insight about their marketing plans and benefits of the technology.

EU Regulatory Roundup, April 2024: EU On Cusp Of Regulatory Shifts Amidst Political Change
There are new EU regulations on medtech’s horizon following votes this month by the European Parliament. There are also unknowns when it comes to future leadership and the direction it will take as industry advocates for much-needed change.

Valuable Health Data Sources For Medtech Industry Come A Step Closer In The EU
The only step that remains now before the European Health Data Space Regulation is approved is sign off by the Council of the EU, due next month. Industry wants to see the new framework carefully aligned with existing EU legilsation.

EU: Good News For IVDs And For Future Transparency Of Medtech Compliance
The IVD industry has long been awaiting a further extension of the deadlines for compliance with the IVD Regulation and for the launch date of the Eudamed database to be brought forward.

UK MHRA Updates Assistive Tech And Borderline Regulations
Device classification themes were uppermost in April for the UK regulator, which issued key guidance in two areas prone to complexities. It also contributed to the MedTech Directorate’s one-year progress report.

EU Experts Want One-Stop-Shop EU Governance That Mimics Best Of Other Jurisdictions
It may be blue sky thinking to surmise how a new EU medtech regulatory governance structure could evolve. But with change on the horizon, experts see exciting opportunities.
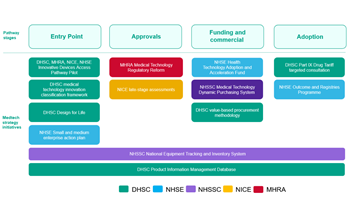
UK Medtech Strategy Sets Out Schedule Of Milestones To FY 2026
Fourteen months on from the release of its inaugural medtech strategy, the UK MedTech Directorate has laid firm foundations and reports progress on initiatives aimed at improving technology adoption. A schedule of ambitious future timelines has also been published.

MAISI: Navigating The 'Valley Of Death' In Medtech Research Translation
Translating research from proof of concept to clinical investigations is a difficult hurdle to overcome. To succeed, researchers need to design their technology for industrial standard manufacturing early on, Anne Vanhoestenberghe, director for the Manufacture of Active Implants and Surgical Instruments (MAISI), told Medtech Insight.

Second EU MDR Notified Body Designated In France
Four years after the designation of the first notified body in France under the Medical Device Regulation, AFNOR Certification has been named too.

EU Regulatory Experts Support Notified Bodies, But Argue For Greater Consistency
Notified bodies have been a pivotal part of the EU medtech regulatory system since it was first launched in the 1990s. Where might they fit within a new medtech regulatory governance structure? Panelists on a recent vodcast grappled with the question.

First Public Discussion On How EU Medtech Regulatory Governance Structure May Evolve
Does the EU need a medtech agency for the first time in its history? Nothing can or should be decided too quickly but five high-profile experts broadly agreed that change is now critical.

First Danish Notified Body Named Under EU's Medical Device Regulation
The EU now has a total of 45 notified bodies under the MDR.

UK Body Proposes Contract To Expedite Clinical Trials
The ownership of all study data would be granted to the non-commercial sponsor, says one of the many provisions proposed in the model investigator-initiated study agreement that the Health Research Authority has released for consultation.

Why Vital EU Guidance For Clinical Investigation Plans Is So Challenging To Read
Errors or omissions are commonplace when sponsors write their clinical investigation plans. Good signposting is urgently needed. Industry expert Maria Donawa gave some useful tips in how to best read the latest EU clinical trials guidance.
You must sign in to use this functionality
Authentication.SignIn.HeadSignInHeader
Email Article
All set! This article has been sent to my@email.address.
All fields are required. For multiple recipients, separate email addresses with a semicolon.
Please Note: Only individuals with an active subscription will be able to access the full article. All other readers will be directed to the abstract and would need to subscribe.