Medtech Insight
Luna Health has raised $23.6m in Series A funding to support regulatory submissions, clinical studies and manufacturing for its insulin patch pump.
Combination products are no longer fringe – they are the “new normal,” consultant Stephen O’Rourke told Medtech Insight. The EU has the expertise to handle the regulatory complexity, but only if connects the dots between silos. That’s the challenge, he said, and the opportunity.
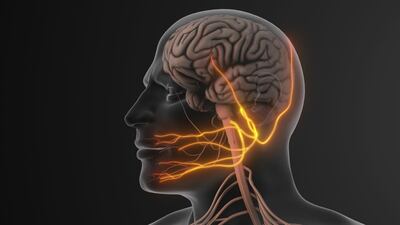
The US FDA has cleared NeuroOne's OneRF trigeminal nerve ablation system for the treatment of trigeminal neuralgia. This minimally invasive method utilizes radio frequency energy to alleviate pain, offering an alternative to traditional medications and surgeries. A fall commercial launch is planned.
AI-driven medtech M&A slowed slightly in volume but tripled in value in early 2025, with buyers focusing on AI-driven diagnostics, surgical tech and analytics. Industry experts expect continued high-value deals as firms defend market share and pursue workflow-enhancing AI.
Philips says the expansion of its facility in Reedsville, PA, will not only boost its capacity to produce AI-enabled ultrasounds that are used in hospitals across the US but will also create 120 well-paying jobs.
A government-backed program is working on improving the ability to run dementia clinical trials in the UK, and access to trials for participants.
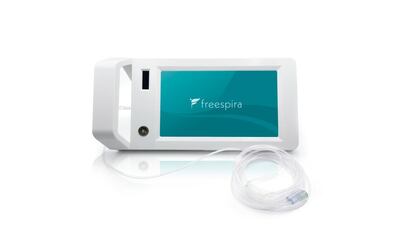
Freespira’s device has been FDA-approved to treat panic disorders and PTSD. CEO Joe Perekupka told Medtech Insight the company’s innovative approach includes patient coaching, insurance partnerships to identify potential users, and lobbying for broader insurance coverage for digital therapeutics.
The US FDA posted three warning letters in July, covering medical supply kits, wearables and orthopedics.
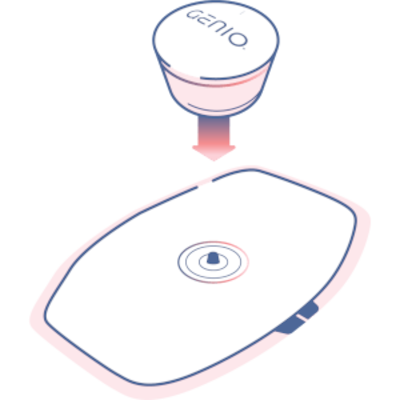
After receiving FDA clearance for its Genio sleep apnea implant, Nyxoah plans a major US rollout despite a patent suit from rival Inspire Medical. Genio offers bilateral nerve stimulation as a CPAP alternative, with strong trial results.
Draeger Medical has recalled certain SafeStar and TwinStar ventilation filters after reports of serious injuries caused by misleading carbon dioxide readings.
The UK device regulator wants to align health institution device exemptions with its evolving policy of agile regulation of medtech in the British market. It asks stakeholders to complete a survey by Sept. 15.
An FDA panel has endorsed the use of dermal fillers for décolletage, but warned of patient safety concerns. The filler can cause complications with future imaging and pregnancy or breastfeeding, panelists said. Regulatory measures and patient studies are recommended for better outcomes.
Synchron is preparing a pilot study of its fully wireless, second-gen brain-computer interface after an ALS patient controlled an iPad solely by thought. If all goes as planned, Synchron’s BCI will move into pivotal trials in 2026.
Apreo Health CEO Karun Naga talks to Medtech Insight about the company’s intentions for its Series B funding. The Breathe-3 clinical trial and early commercialization activities, involving physician education, are top priorities.
An interactive look at recent executive-level company changes and promotions in the medical device and diagnostics industries.
The National Advertising Division (NAD) alerted Agendia to improve disclosure regarding a physician endorsing its MammaPrint test. The physician's ties to Agendia were inadequately revealed in LinkedIn posts.
Just a year and four months after publishing a final rule that would have allowed it to regulate laboratory developed tests as medical devices, the US FDA has rescinded the controversial measure, finally putting an end to the saga.
Exact Sciences has entered into an exclusive licensing agreement with Freenome, stipulating clinical benchmarks and a first-line rating in the USPSTF guidelines. Medtech Insight interviewed screening CMO Paul Limburg about CRC screening and Exact’s strategy on liquid biopsy.
Despite staffing cuts and uncertainty at the FDA, the US still offers EU medtech firms stronger regulatory support, regulatory expert Bassil Akra told Medtech Insight. This is especially valued as EU rules are often viewed as overly stringent, unclear and difficult to follow.
2026 reimbursement calculations for the list of hybrid DRGs – a mechanism to incentivize day cases over inpatient surgery – will be issued in few weeks’ time. The medtech industry has made its demands known.