United States
The US FDA has launched the Regulatory Accelerator to expedite digital health product development. This includes resources like a Resource Index for Innovators, a Medical Device Software Guidance Navigator, and best practices for early meetings.
In his first 100 days as FDA commissioner, Martin Makary has gone from vocal critic to vocal supporter of the agency’s staff, calling them “phenomenal” and praising their dedication—even as morale remains shaken by recent layoffs and restructuring.
The medtech M&A landscape is experiencing a resurgence fueled by significant capital from private equity and venture firms, says Alex Wakefield, CRO of AcuityMD. Building strong relationships with physicians remains crucial in medtech, and defining an exit strategy early is imperative.
The US Department of Health and Human Services has proposed cutting Medicare reimbursement for skin substitutes used in chronic wound care by up to 90%. The change aims to address rising costs but could harm patient access and treatment quality, prompting concerns from industry stakeholders.
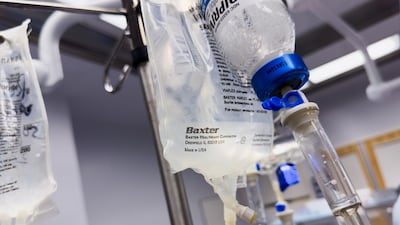
The US FDA says Baxter has notified customers about an issue with its Novum IQ Syringe Pump that is linked to two deaths and multiple injuries.
A federal court in Illinois ruled against SurModics and BC Holdings' request for FDA documents to support a proposed merger. The FTC has challenged the merger, claiming it threatens competition in hydrophilic device coatings. The ruling may indicate the FTC is likely to succeed.
Despite headwinds, executives remain optimistic about growth in Abbott’s medical devices and diabetes divisions and plan several product rollouts, including the Volt PFA catheter for electrophysiology and the dual glucose-ketone sensor CGM.
The exemption, which President Donald Trump announced on July 17, is intended to cut the risk of critical device shortages. Stakeholders say the technology needed to cut emissions to required levels is not yet widely available.
Dexcom has recalled several models of its glucose monitoring receivers due to a speaker glitch that may suppress vital blood sugar alerts. The FDA designated the recall, which affects thousands of devices, as class I.
A recent JAMA column called for the US FDA to create an online database of medical device labeling, arguing that the move would increase transparency and help researchers, among other benefits. Medtech Insight discussed the idea with the paper’s lead author.
A recent FDA warning letter claims a Boston firm that specializes in wearable technology marketed a blood pressure device without agency approval. The company rejects the assertion and says the agency is out of step with federal law.
US Medicare has proposed national coverage of renal denervation for patients with uncontrolled hypertension. The treatment is seeing other advances as well, with Medtronic piloting a longer catheter and a multi-organ approach and a blood test to identify the best candidates fresh on the
The US House of Representatives passes legislation requiring manufacturers of non-flushable wet wipes to label products as ‘non-flushable," a move endorsed by the Personal Care Products Council.
The pulsed-field ablation market is surging, with the UK NHS opening doors and FDA updates for major players. Medtech Insight spoke with Steven Mickelsen, founder of Farapulse (the first clinically approved PFA system), about the sector's growth and his new venture, Field Medical.
Medicare beneficiaries with tricuspid regurgitation will now have access to an innovative treatment from Abbott that offers a minimally invasive alternative to open-heart surgery.
MedTech Europe wants the EU to continue its commitment to reach a negotiated tariff solution with the US and measured restraint amid escalating trade tensions.
A surgeon at Ochsner Health in New Orleans was able to successfully remove a rare and dangerous tumor from a patient’s spine using cutting-edge technology that would have otherwise made the procedure too risky.
The FDA's new Commissioner’s National Priority Voucher program aims to expedite drug approvals significantly. Device firms like XVIVO advocate for a similar initiative for devices, emphasizing the potential for faster reviews and improved patient access to lifesaving technologies.
A recent approval from the US FDA allowing Boston Scientific to broaden its labeling for its pulse field ablation system means more patients with one type of AFib will have access to a promising new treatment.
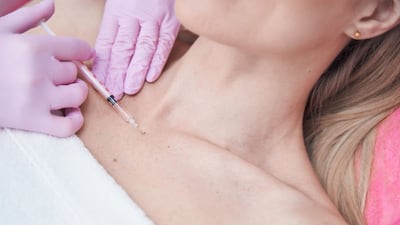
An upcoming US FDA advisory panel meeting will discuss adding a new indication to allow dermal fillers to be used in the upper chest, or décolletage. Plastic surgeons expect this could drive interest in the procedure, which is already performed off-label.