EU member states have issued a consensus statement on the urgent need to build momentum to tackle governance and centralization issues as part of much-needed reform of the EU medtech regulatory system.
A deep-learning AI-based retinal image scanning tool can predict the risk of cardiovascular events over a 10-year period with 70% accuracy. The test is comparable to routine GP health checks, researchers from the University of Dundee have shown.
The pulsed-field ablation market is surging, with the UK NHS opening doors and FDA updates for major players. Medtech Insight spoke with Steven Mickelsen, founder of Farapulse (the first clinically approved PFA system), about the sector's growth and his new venture, Field Medical.
MedTech Europe wants the EU to continue its commitment to reach a negotiated tariff solution with the US and measured restraint amid escalating trade tensions.
The European Commission is focusing on how tiny particles behave to help create an EU industrial “powerhouse.”
Medtech companies require expertise to navigate complex AI regulations and integrate AI in medical software while addressing regulatory challenges, claims expert AI consultant with medtech experience.
Paul Campbell, chief regulatory officer at HealthAI, emphasizes existing regulations for AI in healthcare should not be overlooked as new regulations are developed.
The last steps are taking place leading to the launch of the EU’s medical device database, EUDAMED.
A recent approval from the US FDA allowing Boston Scientific to broaden its labeling for its pulse field ablation system means more patients with one type of AFib will have access to a promising new treatment.
A revised annex to the original decision also outlines expert remuneration limits and introduces further changes.
Thena Capital, the first UK-based early-stage specialist medtech firm, has made its first investment since closing its £50m fund. Medtech Insight spoke with general partner Pamela Walker Geddes to gain insight into Thena Capital’s investment strategy.
Finland has initiated a project to improve the effectiveness of the monitoring of software intended for medical use and to ensure that software placed on its market meets the requirements set for it.
The Milner Institute, the on-campus hub for start-up acceleration at Cambridge University, hosted its annual Pitch Day on July 1. Start-ups Panakeia, PathwayBio and Sentinal4D presented diagnostic technologies.
To succeed in medtech, investors must focus on patient outcomes. Gilde Healthcare says involving patients boosts product effectiveness, access and market success — making it a smart strategy in today’s strained healthcare systems.
Broad participation by EU member states in a new pilot to test a unified procedure for evaluating applications for combined drug and IVD studies shows they recognize its value, says Monique Al, vice-chair of the Clinical Trials Coordination Group.
MedTech Europe is ready to become involved and shape Europe’s Life Sciences Strategy and help drive regulatory simplification from the top to make the EU “the world’s most attractive place for life sciences by 2030.”
The UK government’s blueprint to deliver an NHS "fit for the future" was released on July 3.
The rate at which new documents to support the implementation of EU medtech regulations are issued has slowed of late; but June bucked the trend, with a flurry of activity.
The NHS 10-Year Plan officially released on July 3 will crystalize NICE’s Rules-Based Pathway – a new concept for medtech evaluation that will come with a guarantee of funding – but only for a select number of products. Government to also introduce “innovator passport” to speed innovation into NHS.
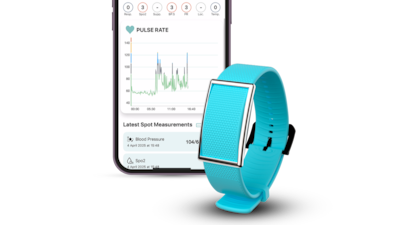
“The Corsano wearable strengthens Medtronic’s acute care and monitoring portfolio,” Marc De Martini, vice president at Medtronic, told Medtech Insight.