Digital Technologies
The European Commission is focusing on how tiny particles behave to help create an EU industrial “powerhouse.”
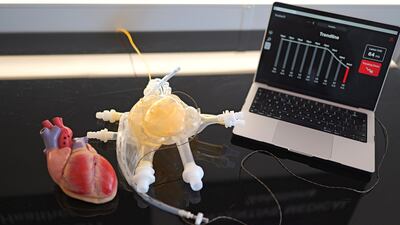
Bivacor aims to be first to the US market with a permanent total artificial heart, starting with use as a bridge to transplant. CMO William Cohn says data from countries with low transplant rates could support pivotal trials and long-term use.
Medtech companies require expertise to navigate complex AI regulations and integrate AI in medical software while addressing regulatory challenges, claims expert AI consultant with medtech experience.
A surgeon at Ochsner Health in New Orleans was able to successfully remove a rare and dangerous tumor from a patient’s spine using cutting-edge technology that would have otherwise made the procedure too risky.
Paul Campbell, chief regulatory officer at HealthAI, emphasizes existing regulations for AI in healthcare should not be overlooked as new regulations are developed.
Researchers at Johns Hopkins have used a robotic system to autonomously perform a key part of gallbladder surgery without a surgeon's hand. Lead author Axel Krieger says it could take five to 10 years before an autonomous robotic system will reach human trials and expects regulatory hurdles.
Joshi joins the parent company of Citeline, home of Medtech Insight, to focus on AI, business harmonization and long-term growth.
Thena Capital, the first UK-based early-stage specialist medtech firm, has made its first investment since closing its £50m fund. Medtech Insight spoke with general partner Pamela Walker Geddes to gain insight into Thena Capital’s investment strategy.
The Milner Institute, the on-campus hub for start-up acceleration at Cambridge University, hosted its annual Pitch Day on July 1. Start-ups Panakeia, PathwayBio and Sentinal4D presented diagnostic technologies.
Corify Care’s CEO said the company aims to submit its 510(k) application to the US FDA by year-end, targeting market clearance in 2026. The company hopes its collaboration with the Mayo Clinic will help speed adoption of ACORYS, a noninvasive mapping technology for use in complex cardiac ablations.
“The Corsano wearable strengthens Medtronic’s acute care and monitoring portfolio,” Marc De Martini, vice president at Medtronic, told Medtech Insight.
The US FDA has issued an updated final guidance document on cybersecurity considerations for medical device manufacturers that replaces a previous final guidance the agency issued in 2023.
Medtronic, Tandem and Beta Bionics are gearing up to bring tubeless patch pumps to market, chasing Insulet’s Omnipod 5, the only FDA-cleared patch pump for type 1 and type 2 diabetes. Analysts expect tubeless models to eventually displace durable pumps.
Backed by £50m of UK government funding, the new research center will delve into neural dynamics to develop novel devices ranging from brain implants to wearables.
Leaders of robotic systems companies Distalmotion, Neocis and Noah Medical discussed success metrics, competition and funding. Institutional investors are focusing on utilization, procedure rates and a clear path to profitability as the IPO window reopens, BTIG analyst Ryan Zimmerman said.
Dxcover sets up new Clinical Laboratory Improvement Amendments (CLIA) lab in Franklin, Tennessee.
Fallouh Healthcare has received £305,050 in grant funding from Innovate UK as part of the European Union’s Eureka Eurostars program. The company aims to detect cardiac tamponade, a condition that affects patients after heart surgery. Currently, there is no way to accurately diagnose the condition.
It’s no secret that Marty Makary and Robert F. Kennedy Jr. have a different take on user fees, with Makary viewing them as a funding necessity and Kennedy as a corrupting influence. But will they find a way to strike a balance during the next MDUFA reauthorization talks so each can claim victory?
Flow Neuroscience's neuromodulation headset for the treatment of depression is approved and marketed in the EU. However, two years on, the company is still waiting for US FDA approval despite being assigned breakthrough device designation.
FDA officials Michelle Tarver and Marty Makary discussed advancements in home healthcare during a podcast. They highlighted the importance of continuous glucose monitors, emphasized home healthcare's benefits for chronic diseases, and mentioned the agency's efforts to streamline device development.