Deals
Roche aims to grow its diagnostics sales by mid to high single digits, said CEO of Roche Diagnostics Matt Sause. The company unveiled the Axelios synthesis and sequencing solution and discussed its “long-term commitment” to China, as well as its business strategy for the next five years.
The consumer genomics firm will operate as a subsidiary, while Regeneron plans to leverage its database for drug discovery and trial design efforts.
An interactive look at medtech and diagnostics deals made during March 2025. Data courtesy of Biomedtracker.
The firms recently announced they will operate as combined company LXE Hearing marketing Eargo’s namesake line and hearX’s Lexie brands. Eargo majority owner Patient Square Capital added $100m to its investment.
Financing
Medtech Insight spoke with Hubert Martens, CEO of Netherlands-based neuromodulation company Salvia Bioelectronics, about the company’s innovative implant for treating chronic migraines, ongoing clinical trials and plans for US clinical trials and commercialization.
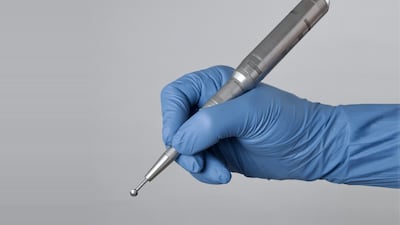
Surgify Medical’s selective drill tip, Surgify Halo, is “an obvious choice for surgeons,” said Boris Hofmann, head of ZEISS Ventures and lead investor in the company’s series A funding round.
German Bionic’s new exoskeleton Exia helps healthcare practitioners, nurses, and other caregivers to lift and move patients by supporting muscle movement and reducing the risk of injury.
Guardant Health introduces a new germline panel test to help guide cancer treatment, assess the risk of secondary cancers in patients and identify family members at risk of cancer.
Investor Eye
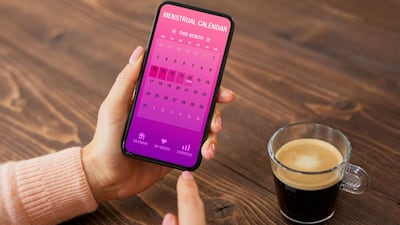
FemTech leaders discussed some of the barriers that remain in unlocking the full potential of the women’s health market during SiS New York last week. They also offered solutions.
Flo Health was the first femtech unicorn to be valued at over $1bn. But the company faced 49 investment rejections before reaching that status, Anna Klepchukova, the company’s chief medical officer, told the LSX World Congress Europe. THENA Capital’s Esther Reynal de St Michel Richardot talked through the firm’s gender-smart investment strategy.
Health tech is at the forefront of the women's health sector, securing 38% of venture capital in 2024. However, panelists at the Anglonordic Life Science Conference held 3 April asserted that a successful therapeutic breakthrough is key to gaining investor confidence and accelerating venture capital.
Sofinnova Partners has played an important role in defining the medtech investment space for the past 50 years, helping bring to market a plethora of life-saving technologies, even when they appear, initially, to be risky.
R&D
Cardiosense has launched a nationwide clinical study, SEISMIC-HF II, to validate its non-invasive, AI-powered technology for monitoring heart failure. The data will be used to file for US regulatory clearance of the Cardiosense heart failure monitoring platform.
Valued at over $1.9bn, ConcertAI is building on its pre-existing multi-agentic AI SaaS solution CARAai to bring life science customers the new Precision suite of applications: Precision Explorer, Precision Trials, Precision GTM and Precision360.
Handheld diagnostics are more powerful, accessible and clinically relevant than ever. Medtech Insight spoke to companies behind such technologies to learn how they work and discuss their commercial models.
Roche aims to grow its diagnostics sales by mid to high single digits, said CEO of Roche Diagnostics Matt Sause. The company unveiled the Axelios synthesis and sequencing solution and discussed its “long-term commitment” to China, as well as its business strategy for the next five years.
Startups & SMEs
Valued at over $1.9bn, ConcertAI is building on its pre-existing multi-agentic AI SaaS solution CARAai to bring life science customers the new Precision suite of applications: Precision Explorer, Precision Trials, Precision GTM and Precision360.
Surgify Medical’s selective drill tip, Surgify Halo, is “an obvious choice for surgeons,” said Boris Hofmann, head of ZEISS Ventures and lead investor in the company’s series A funding round.
German Bionic’s new exoskeleton Exia helps healthcare practitioners, nurses, and other caregivers to lift and move patients by supporting muscle movement and reducing the risk of injury.
TRiCares presented data from the first-in-human study for its tricuspid valve replacement system – Topaz – at EuroPCR 2025 on 22 May.
Strategy
Valued at over $1.9bn, ConcertAI is building on its pre-existing multi-agentic AI SaaS solution CARAai to bring life science customers the new Precision suite of applications: Precision Explorer, Precision Trials, Precision GTM and Precision360.
Roche aims to grow its diagnostics sales by mid to high single digits, said CEO of Roche Diagnostics Matt Sause. The company unveiled the Axelios synthesis and sequencing solution and discussed its “long-term commitment” to China, as well as its business strategy for the next five years.
BD is accelerating transitions of product sourcing for China to avoid tariffs. Historically, products like Vacutainer were exported from Sumter, SC, to China, and Flush was shipped from Columbus, NE.
During MD&M East in Manhattan last week, a panel of experts discussed how the Trump administration’s trade policy is affecting manufacturing and offered some ideas on what manufacturers can do to help mitigate the chaos.