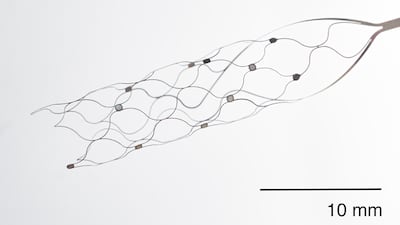
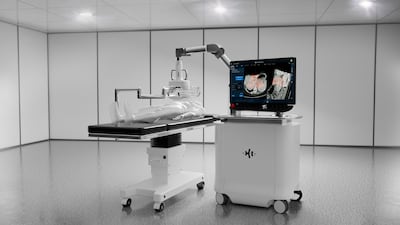
TRiCares presented data from the first-in-human study for its tricuspid valve replacement system – Topaz – at EuroPCR 2025 on 22 May.
A study from Babson Diagnostics published in the peer-reviewed Journal of Applied Laboratory Medicine showed that capillary blood sample volume issues can overcome historical challenges, such as poor quality, through a technique called assay miniaturization.
The UK has issued clearer guidance to help drug and medical device sponsors demonstrate how they intend to include a diverse and relevant range of participants in their clinical trials.
Fujirebio Diagnostics' Alzheimer's disease test, Lumipulse G pTau217/ß-Amyloid 1-42 Plasma Ratio, has been cleared by the US FDA.
Mirvie launched Encompass, a blood test to help identify women over age 35 who are at moderate risk for preeclampsia, and will conduct additional studies to support reimbursements from payers.
Israeli-based SpotitEarly hopes to bring an early cancer-detection test, which uses dogs’ noses to detect compounds in exhaled breath and AI analysis, to US homes in 2026.
King’s College London, Imperial College London and The Alan Turing Institute constructed cardiac digital twins at scale, creating over 3,400, in a new study using UK Biobank data published in Nature Cardiovascular Research on 16 May.
Flo Health was the first femtech unicorn to be valued at over $1bn. But the company faced 49 investment rejections before reaching that status, Anna Klepchukova, the company’s chief medical officer, told the LSX World Congress Europe. THENA Capital’s Esther Reynal de St Michel Richardot talked through the firm’s gender-smart investment strategy.
Apple and Synchron are teaming up to develop technologies that will one day allow people who can’t use their hands or voice to control iPhones, iPads and other Apple devices by using only their thoughts.
The REFLECT studies showed a 78% reduction in cardiovascular disease-related hospitalization for people living with type 1 diabetes with prior low blood sugar episodes.
After publishing encouraging results from first-in-human trials of its brain-computer interface, Axoft announced plans to sell its BCI-enabling material Fleuron to researchers and private organizations for R&D use. The company sees this as a revenue stream and feedback loop to refine its BCI platform designed for safer, longer-lasting brain implants.
Peerbridge Health is preparing to submit its next-generation ECG patch, CorMDx, for US FDA clearance this quarter, with plans to launch in the second half of 2025. The rechargeable device is designed for continuous, real-time heart monitoring from the hospital to home, aiming to detect early signs of heart failure and reduce emergency room visits.
Neurotechnology start-up Subsense came out of stealth with $17m in seed funding to develop a nonsurgical, nanoparticle-based brain-computer interface. Medtech Insight spoke with the firm’s new neurotech lead Cyril Eleftheriou about the technology and its potentially wide applications for treating Parkinson’s, epilepsy, inner speech decoding, and more.
At AAOS, orthopedics players showcased their latest robotic-assisted platforms, power tools, 3D printed technologies and software offerings. This article brings you highlights from interviews Medtech Insight conducted on site with representatives from J&J, Stryker, Materialise and Canary Medical.
Back-to-back meetings at LSX? No time to attend the innovator showcase? Here is what you might have missed from medtech innovators at the LSX World Congress Europe on 29 April.
HistoSonics, which developed a noninvasive technology to destroy tumor cells, reports 90% local tumor control at 12-month follow-up in the #HOPE4Liver Trial. The Edison System, cleared by the US FDA in late 2023, is also being evaluated for kidney and pancreatic tumors. CEO Mike Blue said the medtech is financially secure but watching public markets as it considers an IPO.
Medtronic says results from a recent study on the Hugo robotic-assisted surgery system substantiate its safety and effectiveness for various urological procedures. The company also announced it has submitted the system to the FDA for approval for a urological indication.
Cambridge-based startup Mursla Bio's liquid biopsy test EvoLiver uses extracellular vesicles to detect hepatocellular carcinoma (HCC) in high-risk cirrhotic patients. Medtech Insight sat down with with Mursla Bio CEO Pierre Arsène.
Biolinq plans to use the proceeds of its new venture funding to support US FDA de novo review, automation engineering and commercialization efforts with partners, CEO Rich Yang told Medtech Insight.
AI systems used in healthcare are vulnerable to adversarial cyberattacks, which are a growing concern, said Atif Azad, a professor of AI at Birmingham City University. Azad’s research group has developed a method that trains AI to become more resilient to cyber threats through the use of random image adjustments.