Approvals
A recent approval from the US FDA allowing Boston Scientific to broaden its labeling for its pulse field ablation system means more patients with one type of AFib will have access to a promising new treatment.
A system of pre-regulatory review consultations for innovative drugs and devices in South Korea has contributed to the accelerated development of such products.
The US FDA has announced classifications for five device types, including four diagnostics as well as a hand cream to protect healthcare workers exposed to radiation. Two of the products are newborn screening tests.
Flow Neuroscience's neuromodulation headset for the treatment of depression is approved and marketed in the EU. However, two years on, the company is still waiting for US FDA approval despite being assigned breakthrough device designation.
Medtronic is teaming up with IRCAD North America to build high-tech training and education experiences for surgeons and medical professionals in cardiovascular, neuroscience, and minimally invasive surgical specialties. The move could also further position Medtronic to seize on the growing robotics
Clairity’s "first-in-class" mammography-based AI screening tool, Clairity Breast, provides "equitable risk assessments," expanding access to lifesaving early detection for breast cancer, said company founder Connie Lehman.
Abbott received the US FDA nod for its Tendyne system, offering a minimally invasive alternative to replace the valves of patients with severe mitral valve disease who are at risk for open-heart surgery.
The US FDA has approved the Teal Wand, the first at-home cervical cancer screening device. Capable of detecting preclinical cancer with 96% accuracy, it will launch in California in June and expand nationwide soon after.
NeuroOne is preparing to submit its OneRF Trigeminal Nerve Ablation System to the US FDA for treating trigeminal neuralgia, a chronic facial pain condition. CEO Dave Rosa told Medtech Insight that he expects a possible product launch by fall 2025.
Precision’s recent FDA clearance for a core part of its next-generation wireless brain-computer interface system opens the pathway to a safer, more humane BCI for researchers to use compared to higher-risk intracortical arrays, according to BCI expert Naveen Rao.
“We believe [the SAPIEN M3] launch alongside PASCAL and EVOQUE will help support the company’s target of $2bn in transcatheter mitral and tricuspid therapies sales by 2030,” noted analysts from Leerink Partners.
Dexcom announced it received US clearance for its 15-day CGM, which has a MARD of 8.0% and is expected to launch in the second half of 2025 to allow for integration with insulin pumps.
A concealed blunt-tip needle that can be inserted into the heart's pericardial space to treat cardiac arrhythmias has received FDA clearance, providing an alternative to catheter-based methods.
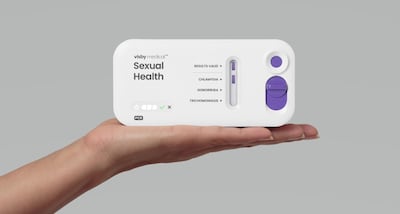
The Visby Medical Women’s Sexual Health Test is the first over-the-counter test cleared by the US Food and Drug Administration to detect chlamydia, gonorrhea and trichomoniasis. It delivers results in about 30 minutes.
The US FDA's clearance of QP-Prostate CAD positions the company to support US health care providers and growing markets for fusion biopsy and focal therapy, said Quibim CEO and founder Angel Alberich-Bayarri.
Tempering expectations on the immediate market impact for the upgraded continuous glucose monitor, Dexcom notes insurance coverage and pump integrations will take time to finalize.
The device, which uses a flexible frame to ease insertion and minimize the dose of copper, is 99% effective in preventing pregnancy. Clinical trial lead David Turok said it represents “a real advance” in contraceptive options.
Medtronic’s US FDA approval for BrainSense aDBS and BrainSense EI software features of the Percept neurostimulator device has quickly followed CE marking in January.
Hologic attains full US Food and Drug Administration 510(k) clearance for its Aptima SARS-CoV-2 assay, which reached market under an Emergency Use Authorization (EUA). Overall, Hologic’s “best-performing” molecular diagnostic business continues to grow despite the decline in revenue for COVID-19 assays and related items.
Boston Scientific has integrated cardiac mapping and pulsed field ablation into a single catheter with the newly EU-approved Farawave Nav Ablation Catheter. Faraview Software also receives CE marking, allowing for visualization of catheter placement when delivering therapy.