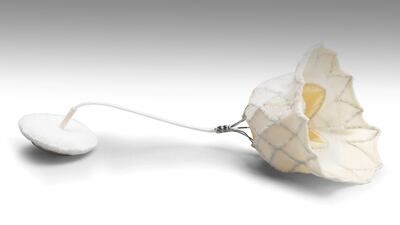
The US Food and Drug Administration approved Edwards Lifesciences’ Evoque tricuspid valve replacement system to improve the “health status” of people with symptomatic severe tricuspid regurgitation.
The approval, announced on 1 February, makes Evoque the first FDA-approved transcatheter tricuspid replacement valve. Evoque earned FDA's breakthrough status in 2019 and a CE mark in October 2023....