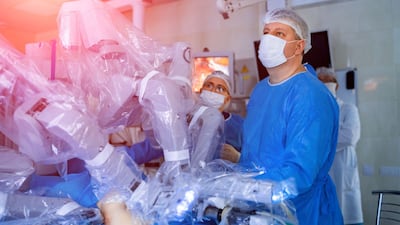
Johnson & Johnson (Pty) Ltd released details of its much-anticipated Ottava robotic surgery platform for soft-tissue procedures during the company’s virtual medical device update on 19 November.
The platform is slated to enter human clinical trials in the second half of 2022 with a possible launch expected...
Welcome to Medtech Insight
Create an account to read this article
Already a subscriber?