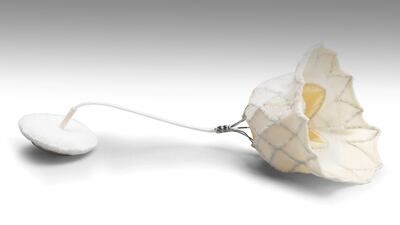
Data from a new study that evaluated the effectiveness of 3D printing and computer modeling in predicting paravalvular leak (PVL) in patients undergoing transcatheter aortic valve replacement (TAVR) has backed the use of the technology for addressing the problem of ill-fitting valves. The data was presented on April 26 at the 41st annual Society for Cardiovascular Angiography and Interventions (SCAI) meeting, which is being held in San Diego through April 28.
In the study, six patients undergoing TAVR for severe calcific aortic stenosis and at risk for PVL had pre-procedure computed...