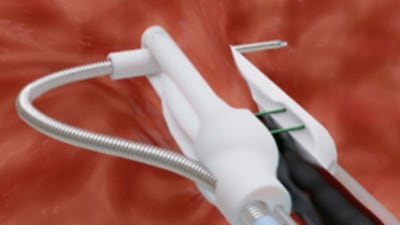
Nevro Corp.'s Senza HF10 SCS became the first spinal cord stimulator with an FDA-approved superiority label and the first approved by FDA to treat chronic pain without creating the tingling sensation called paresthesia.
Approved earlier than expected, the high-frequency stimulation system has the potential to shake up and grow the $1.2 billion spinal...
Welcome to Medtech Insight
Create an account to read this article
Already a subscriber?